IMPORTANT SAFETY INFORMATION
INDICATIONS
IMPORTANT SAFETY INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death (1.6 to 1.7 times) compared to placebo-treated patients. ABILIFY MYCITE is not approved for the treatment of patients with dementia-related psychosis.
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults. Those on antidepressant therapy should be monitored closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber. The safety and effectiveness of ABILIFY MYCITE have not been established in pediatric patients.
Contraindication: Known hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis.
Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse events (e.g., stroke, transient ischemic attack), including fatalities, have been reported in clinical trials of elderly patients with dementia-related psychosis treated with aripiprazole.
Neuroleptic Malignant Syndrome (NMS): NMS is a potentially fatal symptom complex reported in association with administration of antipsychotic drugs, including ABILIFY MYCITE. Clinical signs of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability. Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. Manage NMS with immediate discontinuation of ABILIFY MYCITE, intensive symptomatic treatment, and monitoring.
Tardive Dyskinesia (TD): Risk of TD, and the potential to become irreversible, are believed to increase with duration of treatment and in total cumulative dose of antipsychotic drugs. TD can develop after a relatively brief treatment period, even at low doses, or after discontinuation. If antipsychotic treatment is withdrawn, TD may remit, partially or completely. Prescribing should be consistent with the need to minimize TD.
Metabolic Changes: Atypical antipsychotic drugs have caused metabolic changes including:
- Hyperglycemia/Diabetes Mellitus: Hyperglycemia, in some cases extreme and associated with ketoacidosis, hyperosmolar coma, or death, has been reported in patients treated with atypical antipsychotics including aripiprazole. Patients with diabetes mellitus should be regularly monitored for worsening of glucose control; those with risk factors for diabetes (e.g., obesity, family history of diabetes), should undergo baseline and periodic fasting blood glucose testing. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia should also undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
- Dyslipidemia: Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
- Weight Gain: Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
Pathological Gambling and Other Compulsive Behaviors: Intense urges, particularly for gambling, and the inability to control these urges have been reported while taking aripiprazole. Other compulsive urges have been reported less frequently. Prescribers should ask patients or their caregivers about the development of new or intense compulsive urges. Consider dose reduction or stopping ABILIFY MYCITE if such urges develop.
Orthostatic Hypotension: ABILIFY MYCITE may cause orthostatic hypotension and should be used with caution in patients with known cardiovascular disease, cerebrovascular disease, or conditions which would predispose them to hypotension.
Falls: Antipsychotics may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls causing fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating treatment and recurrently during therapy.
Leukopenia, Neutropenia, and Agranulocytosis: Leukopenia, neutropenia and agranulocytosis have been reported with antipsychotics. Monitor complete blood count in patients with pre-existing low white blood cell count (WBC)/absolute neutrophil count or history of drug-induced leukopenia/neutropenia. Discontinue ABILIFY MYCITE at the first sign of a clinically significant decline in WBC and in severely neutropenic patients.
Seizures: ABILIFY MYCITE should be used with caution in patients with a history of seizures or with conditions that lower the seizure threshold.
Potential for Cognitive and Motor Impairment: ABILIFY MYCITE may impair judgment, thinking, or motor skills. Instruct patients to avoid operating hazardous machinery, including automobiles, until they are certain ABILIFY MYCITE does not affect them adversely.
Body Temperature Regulation: Use ABILIFY MYCITE with caution in patients who may experience conditions that increase body temperature (e.g., strenuous exercise, extreme heat, dehydration, or concomitant use with anticholinergics).
Dysphagia: Esophageal dysmotility and aspiration have been associated with ABILIFY MYCITE. Use caution in patients at risk for aspiration pneumonia.
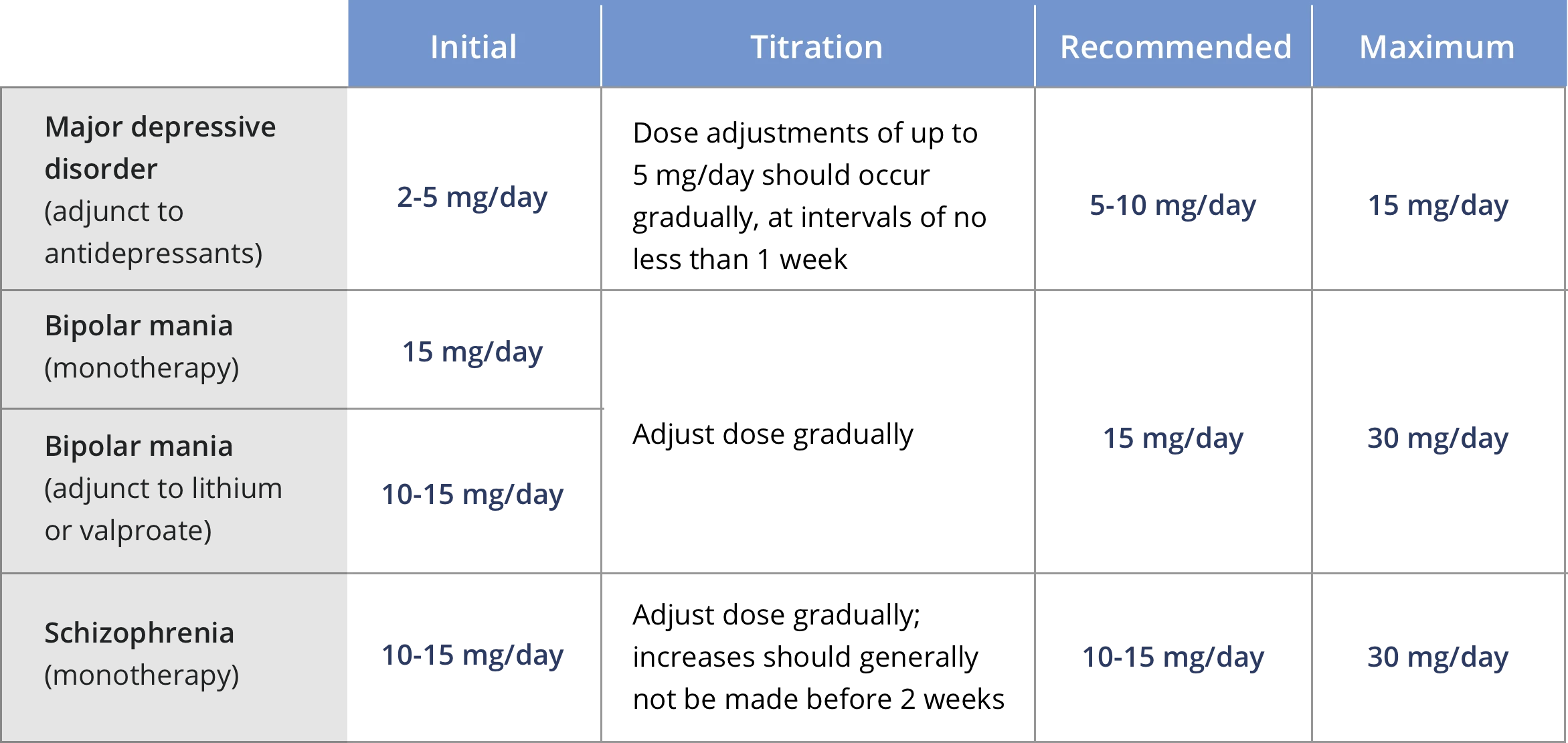
Dosage Adjustments and Cytochrome P450 Considerations: For patients with schizophrenia and bipolar I disorder taking ABILIFY MYCITE who are:
-
Known CYP2D6 poor metabolizers, administer half the recommended dose
-
Known CYP2D6 poor metabolizers taking concomitant strong CYP3A4 inhibitors (e.g., itraconazole, clarithromycin), administer a quarter the recommended dose.
-
Taking strong CYP2D6 (e.g., quinidine, fluoxetine, paroxetine) or CYP3A4 inhibitors, administer half the recommended dose.
-
Taking strong CYP2D6 and CYP3A4 inhibitors, administer a quarter the recommended dose. When co‑administered drug is withdrawn, adjust ABILIFY MYCITE dosage to its original level.
-
Taking strong CYP3A4 inducers (e.g., carbamazepine, rifampin), double recommended dose over 1 to 2 weeks. When co‑administered drug is withdrawn, reduce ABILIFY MYCITE dosage to original level over 1 to 2 weeks.
Commonly Observed Adverse Reactions (incidence ≥5% and at least twice that for placebo) in adult patients:
-
Schizophrenia: akathisia
-
Bipolar mania (monotherapy): akathisia, sedation, restlessness, tremor, and extrapyramidal disorder
-
Bipolar mania (adjunctive therapy with lithium or valproate): akathisia, insomnia, and extrapyramidal disorder
-
Major depressive disorder (adjunctive treatment to antidepressant therapy): akathisia, restlessness, insomnia, constipation, fatigue, and blurred vision
Dystonia: Symptoms of dystonia may occur in susceptible individuals during the first days of treatment and at low doses.
Skin Irritation for MYCITE Patch: Symptoms of skin irritation localized at the site of the MYCITE Patch may occur. In clinical studies, 12.4% of patients (n=61) experienced skin rashes at the site of patch placement.
Pregnancy: Neonates exposed to antipsychotic drugs, including ABILIFY MYCITE, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms. Consider the benefits and risks of ABILIFY MYCITE and possible risks to the fetus when prescribing ABILIFY MYCITE to a pregnant woman. Advise pregnant women of potential fetal risk. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ABILIFY MYCITE during pregnancy. For more information contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://
Lactation: Aripiprazole is present in human breast milk; however, there are insufficient data to assess the amount in human milk, effects on the breastfed infant, or effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for ABILIFY MYCITE and any potential adverse effects on the infant or from the underlying maternal condition.
To report SUSPECTED ADVERSE REACTIONS, contact Otsuka America Pharmaceutical, Inc. at 1-800-438-9927 or FDA at 1-800-FDA-1088 (www.fda.gov/medwatch).
Please see FULL PRESCRIBING INFORMATION, including BOXED WARNING.
INDICATIONS and IMPORTANT SAFETY INFORMATION for ABILIFY MYCITE® (aripiprazole tablets with sensor)
INDICATIONS
ABILIFY MYCITE, a drug-device combination product comprised of aripiprazole tablets embedded with an Ingestible Event Marker (IEM) sensor intended to track drug ingestion, is indicated in adults for the:
- Treatment of schizophrenia
- Treatment of bipolar I disorder as monotherapy and as adjunct to lithium or valproate for:
- Acute treatment of manic and mixed episodes
- Maintenance treatment
- Adjunctive treatment of major depressive disorder
Limitations of Use: ABILIFY MYCITE has not been shown to improve patient compliance or for use in modifying aripiprazole dosage. It should not be used in “real-time” or during an emergency, because detection may be delayed or not occur.